>Our 3rd Report to Stakeholders, 2011-12
Bringing Our Tests to Market
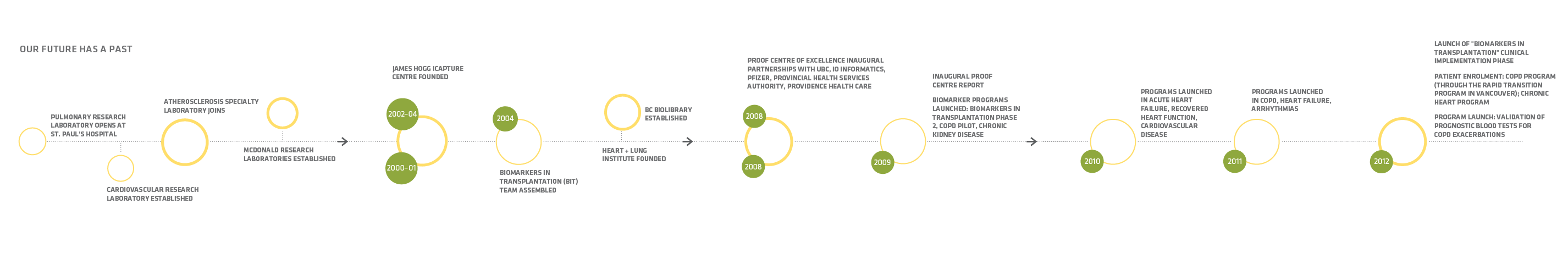
Partnerships and the PROOF Centre’s Development Pipeline








Partnerships and the PROOF Centre’s Development Pipeline








(Expressed in thousands of Canadian dollars)
March 31, 2012, with comparative figures for 2011
| 2012 | 2011 | |
|---|---|---|
| Assets: | ||
| Current assets: | ||
| Cash and cash equivalents | 363 | 1,100 |
| Short-term investments | 4,971 | 7,523 |
| Interest receivable | - | 13 |
| Accounts receivable | 867 | 61 |
| Prepaid expenses and deposits | 59 | 1,050 |
| 6,260 | 9,747 | |
| Capital assets, net of accumulated amortization of | ||
| $3,017(2011 - $2,168) | 614 | 603 |
| 6,874 | 10,350 |
| Liabilities and Net Assets (Deficiency): | ||
| Current liabilities: | ||
| Accounts payable and accrued liabilities | 184 | 125 |
| Deferred operating contributions (note 4) | 6,178 | 9,300 |
| Deferred capital contributions (note 5) | 561 | 548 |
| Net assets: | ||
| Unrestricted | (102) | 322 |
| Invested in capital assets | 53 | 55 |
| (49) | 377 | |
| 6,874 | 10,350 |
(Expressed in thousands of Canadian dollars)
Year ended March 31, 2012, with comparative figures for 2011
| 2012 | 2011 | |
|---|---|---|
| Revenue | ||
| Amortization of deferred operating contributions (note 4) | 5,108 | 3,605 |
| Amortization of deferred capital contributions (note 5) | 799 | 823 |
| Unrestricted contributions | 2,526 | 974 |
| Interest income | 166 | 134 |
| Gain (loss) on investments | (35) | 10 |
| 8,564 | 5,546 | |
| Expenses: | ||
| Core facilities (biomarkers program) | 6,116 | 3,286 | Materials and supplies | 1,455 | 1,243 |
| Equipment | 210 | 127 |
| Administrative salaries | 404 | 346 |
| Administrative operations | 202 | 108 |
| Hosting conferences | 7 | 53 |
| Attending conferences | 169 | 153 |
| Other knowledge dissemination | 43 | 53 | Market studies and business development | 304 | 152 |
| Intellectual property | 79 | 218 |
| Space improvements | 1 | 1 |
| 8,990 | 5,740 | |
| Deficiency of revenue over expenses | (426) | (194) |
(Expressed in thousands of Canadian dollars)
Year ended March 31, 2012, with comparative figures for 2011
| Invested in capital assets | Unrestricted | 2012 | 2011 | |
|---|---|---|---|---|
| Balance, beginning of year | 55 | 322 | 377 | 371 |
| Deficiency of revenue over expenses | (50) | (376) | (426) | (194) |
| Transfer to invested in capital assets | 48 | (48) | - | - |
| Balance, end of year | 53 | (102) | (49) | 377 |
(Expressed in thousands of Canadian dollars)
Year ended March 31, 2012, with comparative figures for 2011
| 2012 | 2011 | Cash provided by (used in): |
|---|---|---|
| Operations: | ||
| Deficiency of revenue over expenses | (426) | (194) |
| Amortization of capital assets | 849 | 873 |
| Amortization of deferred capital contributions | (799) | (823) |
| Gain (loss) on investments | 35 | (10) |
| (341) | (154) | |
| Changes in non-cash operating working capital items: | ||
| Interest receivable | 13 | 104 |
| Accounts receivable | (806) | 4 |
| Prepaid expenses and deposits | 991 | (488) |
| Accounts payable and accrued liabilities | 59 | (752) |
| Deferred operating contributions | (3,122) | (2,838) |
| (3,206) | (4,124) | |
| Investments: | ||
| Additions to capital assets | (48) | (48) |
| Additions (disposals) of short-term investments | 2,517 | (2,313) |
| 2,469 | (2,361) | |
| Decrease in cash and cash equivalents | (737) | (6,485) |
| Cash and cash equivalents, beginning of year | 1,100 | 7,585 |
| Cash and cash equivalents, end of year | 363 | 1,100 |
| Supplemental information: | ||
| Cash received for interest | 179 | 238 |
| Non-cash transactions: | ||
| In-kind donation of capital assets | 812 | 797 |
Dr. George Schreiner,
Chairman of the Board, CEO, Cardero Therapeutics Inc.
Dr. Bruce McManus,
Director, PROOF Centre of Excellence; Co-Director, Institute for Heart + Lung Health; Professor, Department of Pathology & Laboratory Medicine, Providence Health Care and University of British Columbia
Dr. Donald Brooks,
Associate VP Research & International, Director of Support Programs to Advance Research Capacity, University of British Columbia
Ms. Katherine Gibson,
Founding and Managing Director, Helio Consulting Inc.
Mr. Frank Holler,
CEO & Partner, Lions Capital Corporation
Dr. Seigo Izumo,
Independent Consultant
Dr. Heather Manson,
Director of Health Promotion, Chronic Disease and Injury Prevention, Ontario Agency for Health Protection and Promotion
Mr. Bruce Milley,
President, MJC Business Services Inc.
Dr. Volker Pfahlert,
Independent Consultant, Former Head, Roche Centralized Diagnostics
Dr. Bernard Prigent,
Vice-President and Medical Director, Pfizer Canada
Mr. Richard Rees
CEO, Institute of Chartered Accountants of BC
Mr. Carl Roy,
Senior VP, Provincial Health Services Authority and Executive Officer, Emergency Health Services Commission and the BC Ambulance Service
Work on our Biomarkers in Transplantation (BiT) initiative will lead to improved monitoring for patients who have had a heart or kidney transplant using blood tests. The PROOF Centre team has developed blood-based gene and protein marker sets that predict and monitor absence of acute rejection in heart and kidney transplant patients. The gene and protein signatures provided appropriate performance in the international trial. The clinical laboratory assays are currently being developed and will be established first in the clinical laboratory of St. Paul’s Hospital. Our goal in 2013 is to evaluate the performance of these blood tests in real time by working with doctors and their transplant care teams in clinics in Vancouver, BC.
The goal of our COPD program is to identify sets of blood-based gene and protein biomarkers to enable a physician to determine who will experience frequent exacerbation episodes (or “lung attacks”) and who will not, thus allowing more effective management and reduced hospital visits and costs.
In the last year the PROOF Centre team has discovered gene and protein panels to identify patients who will develop multiple exacerbation episodes. We have also worked with health economists and published a paper showing the economic and health benefits of introducing a diagnostic test for COPD exacerbations into the healthcare system. As we prepare to validate the content of these new blood tests, we have developed new partnerships with Siemens Healthcare, Providence Health Care and the Providence Health Care Research Institute.
Chronic heart failure is a progressive disease that occurs when the heart is unable to fill with blood (diastolic failure) and/or pump blood sufficiently (systolic failure). Each condition requires a different management strategy. However, current diagnostic tools, beyond ultrasound in specialists’ hands, are unable to differentiate systolic and diastolic heart failure resulting in inadequate treatment. The PROOF Centre is working to develop a blood test to diagnose diastolic and systolic heart failure and a blood test to monitor the patient’s response to their therapy. As a simple blood test, this test can be performed by family physicians and used in remote geographical locations.
Patients with chronic kidney disease (CKD) can experience slow progression of the disease while others will decline rapidly. However, physicians currently have a limited ability to predict individual patient outcomes and treat them accordingly. We are working to develop blood tests that will help assess the likelihood of CKD progression in individual patients and to monitor their response to therapy. We are exploring the effectiveness of combining these genes and proteins with clinical and demographic data to better predict CKD progression. Once we have decided upon the most promising biomarker panels, we will test them in a new set of patients.
Acute Heart Failure (AHF) is a life-threatening condition where the heart does not function. Patients with AHF may require mechanical ventricular assist devices (often called mechanical heart pumps) or cardiac transplantation, or even face death. Our team has completed enrollment for the first phase of a program developing blood tests to help clinicians manage patients with a ventricular assist device and aid in clinical decision making.
The PROOF Centre is working with AllerGen NCE (Allergy, Genes and Environment Network of Centres of Excellence) to develop blood tests that will speed up clinical trials investigating the efficacy of drugs treating allergic disease. Allergic diseases place tremendous psychosocial and economic burdens on Canadians and the healthcare system, costing up to $15 billion a year in emergency department visits, medications and productivity loss. The team will work to develop a blood test that will predict and diagnose chronic inflammatory responses in allergic asthmatic adults, allowing for effective monitoring of promising therapeutics.
The PROOF Centre has teamed up with IO Informatics to develop a web-based software application for clinicians to use on hand-held devices or other technology. The application will be used with the PROOF Centre-developed blood tests and provide clinicians with an overall score indicating patient risk level, along with the associated clinical recommendations to help guide clinical decision-making.
In a collaborative service agreement with Adiga Life Sciences, the PROOF Centre is helping to develop blood signatures to monitor for the effectiveness of novel vaccines for allergic rhinitis.
Imparting knowledge and providing a stimulating and supportive environment for continuous learning is a key foundation to the success of the PROOF Centre. From visiting scholars, to postdoctoral fellows, graduate students, and undergraduate students, the PROOF Centre provides its trainees with in-depth skills and experiences that span the biological, technological, computational, business, and societal activities involved in developing and commercializing new blood tests. We have involved more than 20 trainees in PROOF Centre activities to date.